Classification Criteria and Treatment Guidelines
Major advances in the diagnosis, classification, and understanding of axial spondyloarthritis (axSpA) have been made within the last few years, which have led to the successful introduction of new targeted therapies for the treatment of axSpA. Optimal treatment of axSpA requires a combination of nonpharmacological and pharmacological treatments. Nonpharmacological strategies involve physical therapy, education, and lifestyle and behavioral changes. Pharmacologic treatments for axSpA include nonsteroidal antiinflammatory drugs (NSAIDs), tumor necrosis factor (TNF) inhibitors, interleukin-17A (IL-17A) inhibitors, and Janus kinase (JAK) inhibitors.1
Figure 1: Treatment Recommendations for Active AS1

The first-line treatment option for patients with symptomatic axSpA are NSAIDs together with physical therapy and patient education. Therapy with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) such as sulfasalazine may have some beneficial effect in patients with peripheral joint involvement but are generally not effective in the majority of patients with axial involvement.1
Up to 50% of patients fail to achieve a clinically significant response to NSAIDs.2 Patients with a poor response, contraindications, or intolerance to NSAIDs should be treated with a biologic. There are 5 TNF inhibitors—infliximab, etanercept, adalimumab, golimumab, and certolizumab pegol—that are approved in the United States and European Union for the treatment of AS. These agents, with the exception of infliximab, are also approved for the management of nr-axSpA.1
Figure 2: Current Approved Biologics for the Management of AxSpA3
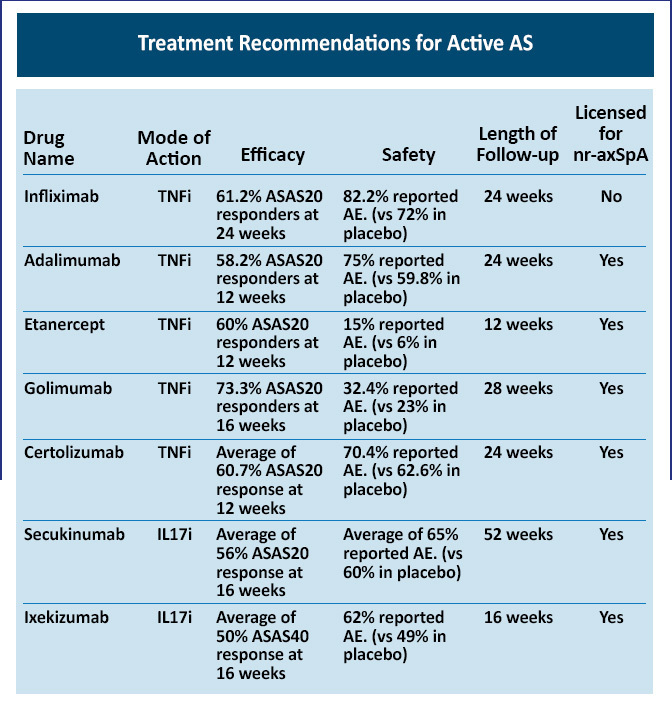
Currently, two IL-17 inhibitors are approved by the US Food and Drug Administration for the treatment of adults with active AS or active nr-axSpA with objective signs of inflammation in the United States: secukinumab and ixekizumab. Tofacitinib, a JAK inhibitor, is approved for the management of AS in patients who fail to respond to any biologic therapy.
The phase III MEASURE 1 and MEASURE 2 trials evaluated the efficacy and safety of secukinumab in patients with active AS. In the MEASURE 1 trial, ASAS20 response rates at Week 16 were 61%, 60%, and 29% for subcutaneous secukinumab 150 mg, 75 mg, and placebo, respectively (P< .001 for both comparisons). Rates in the MEASURE 2 trial were 61%, 41%, and 28% with secukinumab 150 mg, 75 mg, and placebo. Improvements were sustained through Week 52.4 In the PREVENT trial of patients with nr-axSpA who were predominantly naïve to TNF inhibitor therapy, ASAS40 at Week 16 was achieved by 41.5% of patients who received secukinumab 150 mg with loading doses, 42.2% of those who received secukinumab 150 mg without loading doses, and 29.2% of those in the placebo group (P< .05 for both comparisons).5
Ixekizumab is another IL-17 inhibitor treatment option. The COAST-V and COAST-W trials investigated the efficacy and safety of ixekizumab in patients with AS. In COAST-V, patients with no previous history of biologic therapy were randomized to ixekizumab every 2 weeks or every 4 weeks, adalimumab, or placebo. At Week 16, more patients achieved ASAS40 with ixekizumab every 2 weeks (52%; P< .0001), ixekizumab every 4 weeks (48%; P< .0001), and adalimumab (36%; P= .0053) compared with placebo (18%).6 In the COAST-W trial of patients who failed to respond to TNF inhibitor therapy, significantly higher proportions of patients receiving ixekizumab every 2 weeks (30.6%) or every 4 weeks (25.4%) had achieved ASAS40 response versus the placebo group (12.5%), with statistically significant differences reported as early as Week 1 with ixekizumab therapy.7
In the COAST-X trial of adults with active nr-axSpA who failed to respond adequately to NSAIDs, ixekizumab therapy was associated with a significantly greater proportion of patients achieving an ASAS40 response at Week 16 compared to placebo (35% with ixekizumab every 4 weeks, 40% with ixekizumab every 2 weeks, and 19% with placebo). At Week 52, 30% and 31% of patients receiving ixekizumab every 4 weeks and every 2 weeks, respectively, achieved ASAS40 compared to 13% with placebo.8
For patients with AS who fail to respond to any biologic therapy, the JAK inhibitor tofacitinib is a treatment option. In a phase 3 trial of adults with active AS and an inadequate response or intolerance to 2 or more NSAIDs, the ASAS40 response rate at Week 16 was significantly greater with tofacitinib vs placebo (40.6% vs 12.5%; P< .0001).9
References
- Ward MM, Deodhar A, Gensler L, et al. 2019 update of the American College of Rheumatology/ Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Care Res (Hoboken). 2019;71:1285-1299.
- Cheung PP. Anti-IL-17A in axial spondyloarthritis – where are we at? Front Med (Lausanne). 2017;4:1.
- Tahir H, Byravan S, Fardanesh A, et al. Promising treatment options for axial spondyloarthritis: an overview of experimental pharmacological agents. J Exp Pharmacol. 2021;13:627-635.
- Baeten D, Sieper J, Braun J, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med. 2015;373:2534-2548.
- Deodhar A, Blanco R, Dokoupilova E, et al. Improvement of signs and symptoms of nonradiographic axial spondyloarthritis in patients treated with secukinumab: primary results of a randomized, placebo-controlled phase III study. Arthritis Rheumatol. 2021;73:110-120.
- van der Heijde D, Cheng-Cheng Wei J, Dougados M, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active controlled and placebo-controlled trial. Lancet. 2018;392:2441-2451.
- Deodhar A, Poddubnyy D, Pacheco-Tena C, et al. Efficacy and safety of ixekizumab in the treatment of radiographic axial spondyloarthritis: sixteen-week results from a phase III randomized, double-blind, placebo-controlled trial in patients with prior inadequate response to or intolerance to tumor necrosis factor inhibitors. Arthritis Rheumatol. 2019;71:599-611.
- Deodhar A, van der Heijde D, Gensler L, et al. Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial. 2020;395:53-64.
- Deodhar A, Sliwinska-Stanczyk P, Xu H, et al. Tofacitinib for the treatment of ankylosing spondylitis: a phase III, randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2021;80:1004-1013.










