Pathophysiology
Axial spondyloarthritis (axSpA) is an immune-mediated condition characterized by chronic inflammation of the sacroiliac joints (SIJs) and axial skeleton. Inflammation is thought to originate at sites where ligaments and tendons attach to bones (entheses), leading to erosions and new bone formation by mechanisms that are not entirely clear.1,2 For over a decade, the dominant pathogenic paradigm has centered on the dominance of the T helper cell (Th) 1 pathway and tumor necrosis factor alpha (TNF-α).1 Several pathways have been implicated in the development of axSpA, including one focusing on human leukocyte antigen (HLA)-B27, which is a class I major histocompatibility complex molecule. It has been suggested that HLA-B27 may present neoantigens to T cells, resulting in the release of various cytokines that include interleukin (IL)-17 and IL-23. IL-17, in particular, is a proinflammatory cytokine, and the discovery of IL-23 and other members of the IL-17 pathway has led to the consideration of these cytokines having a significant role in the immunopathogenesis of axSpA.1,2
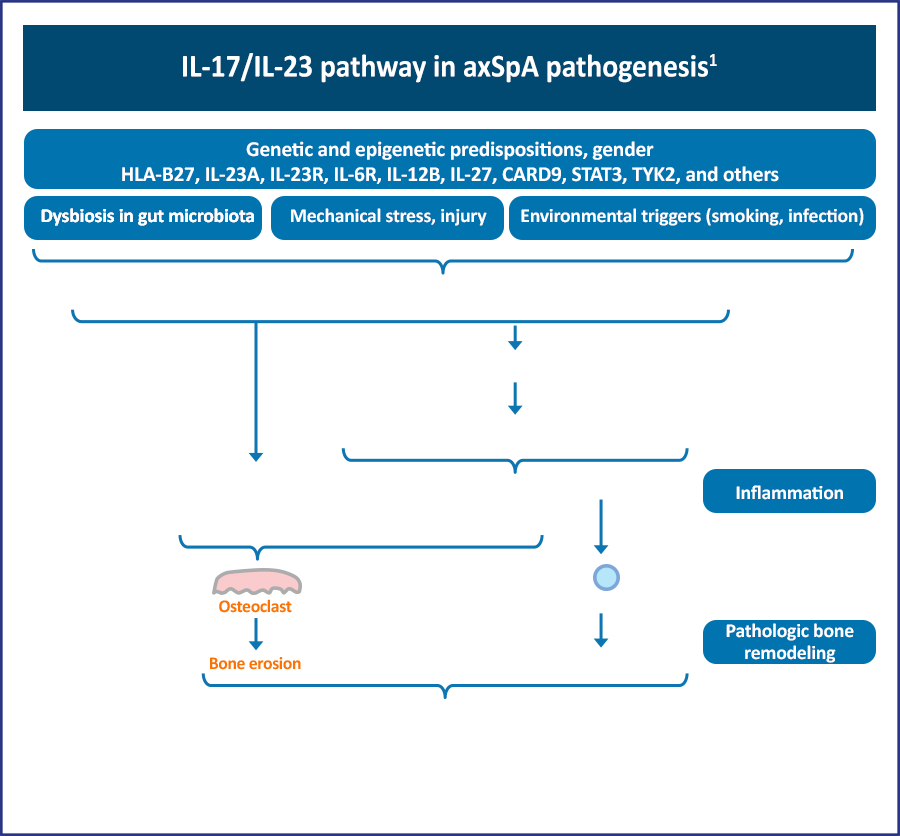
The increased frequency of Th17 cells in these conditions underscores the concept that these disorders can be viewed on a spectrum. Reports of dysbiosis in the gut microbiome of AS patients support previous work indicating a possible causal relationship between altered gut flora, ileocolonic inflammation, and axSpA.1
References
- Paine A, Ritchlin CT. Targeting the interleukin-23/17 axis in axial spondyloarthritis. Curr Opin Rheumatol. 2016;28:359-367.
- Torgutalp M, Poddubnyy D. IL-17 inhibition in axial spondyloarthritis: current and future perspectives. Expert Opin Biol Ther. 2019;19:631-641.










